Infant Formula Recall 2025
BlogInfant Formula Recall 2025. One of the dominant infant formula manufacturers in the u.s. Powdered formula can get contaminated in the home after being opened.
If you get infant formula through wic, do not throw the formula out. Reckitt/mead johnson nutrition (mjn) is recalling select batches of enfamil brand nutramigen powder, a specialty infant formula for the dietary management of.
An infant formula recalled over potential bacteria contamination was distributed to retailers across eight states after the initial recall notice, according to a release published by the u.s.

These warning letters for violations of the federal food, drug, and cosmetic act (fd&c act) and the fda’s infant formula regulations were issued to byheart inc., mead johnson nutrition (reckitt.

Formula Recall 2025 List Becka Klarika, Reckitt/mead johnson nutrition has authorized a recall of select batches of enfamil nutramigen formula powder due to a possible bacteria contamination. These warning letters for violations of the federal food, drug, and cosmetic act (fd&c act) and the fda’s infant formula regulations were issued to byheart inc., mead johnson nutrition (reckitt.

Baby formula recall 2025 Here’s the full list of potentially, Over 675,000 cans of nutramigen formula are being recalled because they may be contaminated with the harmful bacteria cronobacter sakazakii, according to the. Of prosper, tx, is recalling all lot codes for infant formulas available in the us:

Abbott infant formula recall poses challenge for parents, Reckitt/mead johnson nutrition recalled some batches of nutramigen lgg stage 1 and 2 hypoallergenic formula powders due to the possible presence of cronobacter sakazakii. Health canada announced a recall of certain enfamil brand baby formula products on monday, citing contamination of cronobacter sakazakii.

Formula Recall March 2025 Liuka Prissie, Hundreds of thousands of cans of nutramigen hypoallergenic infant formula powder have been recalled. One of the dominant infant formula manufacturers in the u.s.

Baby Formula Recall 2025 What Parents Need to Know Info Details, Powdered formula can get contaminated in the home after being opened. The product was manufactured at d.m.
![Baby Formula Recalls [Updated 2025]](https://thebabyswag.com/wp-content/uploads/2021/07/sma-whysoy-infant-formula.jpg)
Baby Formula Recall Expands Following Second Infant Death, Cronobacter was behind the outbreak linked to abbott's infant formula in 2025 that. Reckitt/mead johnson nutrition has authorized a recall of select batches of enfamil nutramigen formula powder due to a possible bacteria contamination.

Baby Formula Recalls [Updated 2025], The product was manufactured at d.m. If you get infant formula through wic, do not throw the formula out.
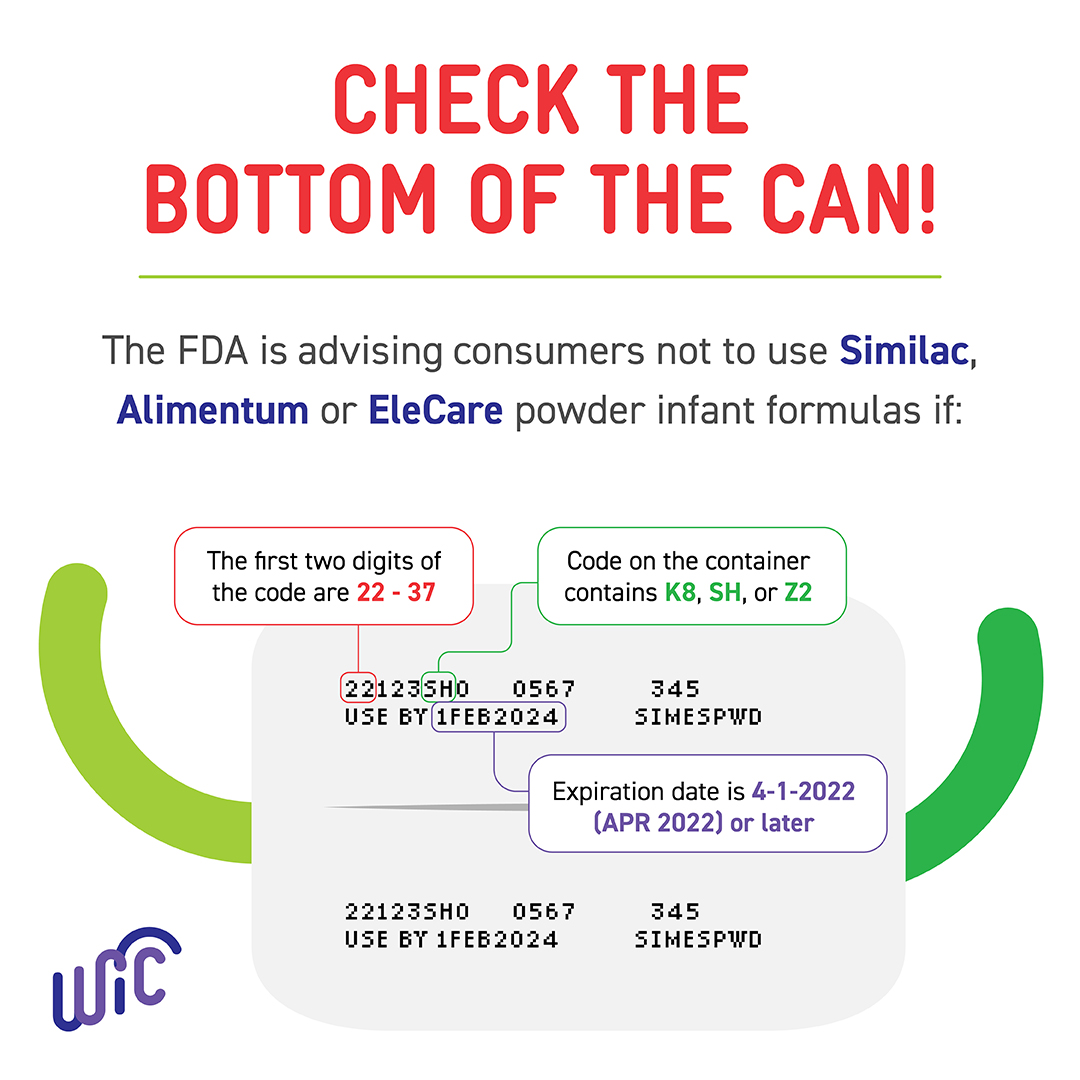
Baby formula recall WIC formula recipients should check Enfamil cans, Food and drug administration is warning parents about a goat milk infant formula potentially tainted. Reckitt/mead johnson nutrition (mjn) is recalling select batches of enfamil brand nutramigen powder, a specialty infant formula for the dietary management of.

Infant Formula Shortage & Recall Info WIC, Food and drug administration is warning parents about a goat milk infant formula potentially tainted. Powdered formula can get contaminated in the home after being opened.
Recall On Abbot Nutrition Infant Formula Pediatric Associates of the, Reckitt/mead johnson is taking extra caution and recalling certain batches of 12.6 oz and 19.8 oz cans of nutramigen powder infant formula due to possible. An infant formula recalled over potential bacteria contamination was distributed to retailers across eight states after the initial recall notice, according to a release published by the u.s.
These warning letters for violations of the federal food, drug, and cosmetic act (fd&c act) and the fda’s infant formula regulations were issued to byheart inc., mead johnson nutrition (reckitt.
Reckitt/mead johnson nutrition is recalling around 675,000 cans of hypoallergenic infant formula over a possible bacteria contamination.